Experiments are preferably conducted in microcosm systems enabling adjustable water tension and full control of ingoing/outgoing compounds and boundary conditions in order to gain mass and energy balances including CO2 fluxes. For examples see, e.g. Poll et al. (2010), Richter et al. (2019). Calorimetric measurements can be performed in heat-protected systems with sensitive temperature measurements (megacalorimeters/cement calorimeters) or bomb / cement calorimeters (Maskow, 2013). This can be combined with techniques such as barometric process separation (Ingwersen et al., 1999; Ingwersen et al., 2008) or using alternatively a diffusion chamber-based approach (Bollmann et al., 2007). Incubation systems should have the following features:
- Closed systems with defined soil-volume to headspace ratios and the option to detect changes in heat production.
- Complete coverage of C balance (solids, liquids, gas) and options for determination of matter and energy distributions to various products (fluxes).
- Control/adjustment of boundary conditions. Standard conditions: see general soil incubation rules below.
- Enable time-resolved (kinetics) and/or spatially highly resolved sampling.
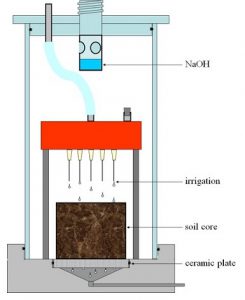
Example for a possible incubation system. Microcosm according to Poll et al. (2010)
Variation of boundary conditions such as temperature, moisture, redox potential and resulting reactions can be used as an experimental approach. Respective details have to be defined by individual projects.
References
Bollmann A., Lewis K., Epstein S.S., 2007. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl. Environ. Microbiol. 73, 6386-6390.
Ingwersen J., Butterbach-Bahl K., Gasche R., Richter O., Papen H., 1999. Barometric process separations: New method for quantifying nitrification, denitrification, and nitrous oxide sources in soils. Soil Sci. Soc. Am. J. 63, 117-128.
Ingwersen J., Schwarz U., Stange C.F., Ju X., Streck T., 2008. Shortcomings in the commercialized barometric process separation measuring system. Soil Sci. Soc. Am. J. 72, 135-142.
Maskow T., 2013. Miniaturization of calorimetry: strengths and weaknesses for bioprocess monitoring and control. in: von Stockar U. (Ed.), Biothermodynamics – the Role of Thermodynamics in Biochemical Engineering. EFPL Press, Lausanne, Switzerland, pp. 423-442.
Poll C., Pagel H., Devers-Lamrani M., Martin-Laurent F., Ingwersen J., Streck T., Kandeler E., 2010. Regulation of bacterial and fungal MCPA degradation at the soil-litter interface. Soil Biol. Biochem. 42, 1879-1887.
Richter A., Kern T., Wolf S., Struck U., Ruess L., 2019. Trophic and non-trophic interactions in binary links affect carbon flow in the soil micro-food web. Soil Biol. Biochem. 135, 239-247.
General soil incubation rules within SPP 2322 SoilSystems
- according to OECD test no. 307 – Aerobic and Anaerobic Transformation in Soil with labelled compounds
- soil sieved to ≤ 2 mm
- temperature 20°C
- substrate supplementation: max. 3-5 fold microbial biomass of the soil (related to C or to energy contents)
- small systems with destructive sampling* or large systems with repeated sampling (→ amount of soil depending on the sampling, > 50% of initial soil mass remaining after last sampling)
- airtight system
- CO2 absorption inside or via gas flux sampling outside
- atmospheric O2 contents, gas space large enough to maintain >15% O2 throughout the incubation
- ≥ 10-15 ml air space per g soil
- maximum layer thickness: 5 cm
- try to reach bulk density 1.1 g cm-3 * (tapping the soil several times, see details in the packing and watering protocol for SPP2322 soils by Schlüter et al. 2022)
- water potential adjusted to pF 2.5 (45% water filled pore space)
- soil pre-incubation with water adjustment for 10 d (essential) at 20°C
- sample storage before incubation start:
- ideal: 4°C, field moisture, not sealed à air exchange, preferably no longer than 60 days (incl. pre-incubation), in particular for all experiments with DNA and RNA analyses
- if not possible due to organizational issues: projects dealing with higher trophic levels (incl. all Core Projects) air-dry soil (optional for appropriate other goals of individual projects: frozen at -20°C )
- ≥ 2 replicates
- ≥ 2 replicates
- maximum incubation time: 90 d (for calorimetric measurements: max. 7 d)
* bulk density can only be adjusted in incubation systems with destructive sampling; this means multiple sampling in bigger systems does not allow bulk density adjustment